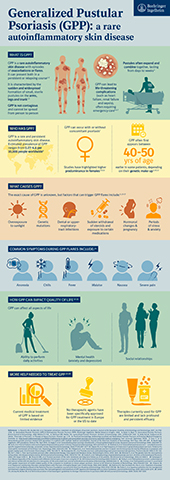
“GPP flares may appear suddenly, intensify quickly, and can be life-threatening if left untreated, leaving those affected feeling anxious and uncertain about their future” said Carinne Brouillon, Member of the Board of Managing Directors and Head of Human Pharma at Boehringer Ingelheim. “The FDA’s recognition of the urgent need for preventing GPP flares is a major step towards empowering people living with the condition to plan critical moments in their lives, despite their disease.”
This designation follows the Center for Drug Evaluation (CDE) of China National Medical Products Administration (NMPA), who also recently awarded spesolimab a BTD for the prevention of GPP flares.
The U.S. FDA and China’s NMPA granted these designations based on the topline results from the EFFISAYIL™ 2 trial which studied whether long-term treatment with the antibody spesolimab helps prevent GPP flares in adolescents and adults with GPP up to 48 weeks.1,2,3 Safety data were in line with previously conducted clinical trials with spesolimab. Data from the trial will be presented at the 25th World Congress of Dermatology 2023 in early July.
About spesolimab
Spesolimab is a novel, humanized, selective antibody that blocks the activation of the interleukin-36 receptor (IL-36R), a signaling pathway within the immune system shown to be involved in the pathogenesis of several autoinflammatory diseases, including GPP.4,6,11 Spesolimab has been approved by regulatory authorities in almost 40 countries including the US, Japan, Mainland China, and the European Union to treat GPP flares in adults.8,9
It is the first approved treatment for GPP flares that specifically targets the IL-36 pathway and that has been evaluated in a statistically powered, randomized, placebo-controlled trial.11 Spesolimab is also under investigation for the treatment of other IL-36 mediated skin diseases.10
About the EFFISAYILTM clinical trial program
The EFFISAYIL™ clinical trial program includes:
- EFFISAYIL™ 1: A Phase II study that demonstrated treatment with a single intravenous dose of spesolimab achieved rapid pustular and skin clearance in patients with GPP flares, sustained over 12 weeks.11 These results supported the approval of spesolimab (SPEVIGO®) as the first specific treatment for GPP flares in adults in major markets.8,9,11
- EFFISAYIL™ 2: A multicenter, randomized, double-blind, placebo-controlled Phase IIb study evaluating the efficacy and safety of maintenance treatment with subcutaneous (SC) spesolimab for the prevention of GPP flares and sustained control of GPP symptoms in adolescents and adults.1,2,3
- EFFISAYIL™ ON: An open-label, extension study to evaluate the long-term safety and efficacy of spesolimab in patients with GPP, who have completed previous spesolimab trials.5
For the full press release including ‘Notes to Editors’ and references please visit: press release